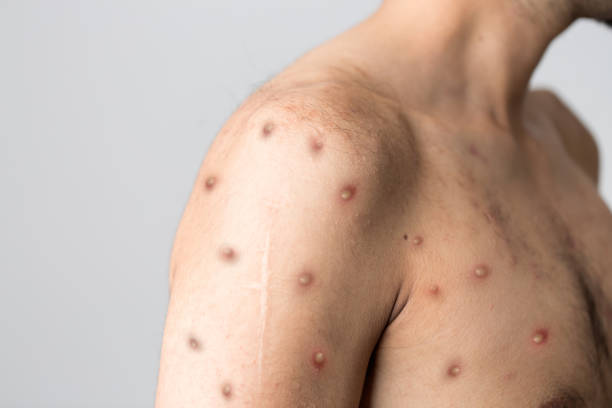
The Africa Centres for Disease Control and Prevention (Africa CDC) has reported a total of 21,466 Mpox cases and 591 deaths in 13 African countries since the beginning of 2024. The outbreak has affected Burundi, Cameroon, Central African Republic, Congo, Côte d’Ivoire, Democratic Republic of Congo, Gabon, Liberia, Kenya, Rwanda, South Africa, Uganda, and Nigeria. Gabon has recently confirmed its first case, while Sierra Leone and Malawi are testing suspected cases.
Dr. Jean Kaseya, Director General of Africa CDC, emphasized the need for a comprehensive approach to managing the Mpox outbreak in a letter addressed to health ministers across African Union member states. He stressed the importance of approving Mpox vaccines for use across the continent, despite global shortages and limited manufacturing capacity.
Kaseya highlighted that from January 1, 2024, to August 23, 2024, there have been 3,350 confirmed cases and 18,116 suspected cases, with a case fatality rate of 2.9%. He called for a unified response and warned that relying solely on laboratory tests can be misleading. A negative test result does not necessarily rule out Mpox, as the disease’s detection can be influenced by factors such as the timing of sample collection and viral load.
The Africa CDC has consulted with international health bodies, including the US CDC, China CDC, Europe CDC, and WHO, to advocate for a holistic approach that integrates clinical assessment and epidemiological data alongside lab results. Kaseya also pointed out that Mpox tests may yield false negatives due to variations in viral strains, sample collection timing, and immune system responses.
Currently, only Nigeria, South Africa, and the Democratic Republic of Congo have approved Mpox vaccines. Kaseya urged other countries to follow suit to ensure equitable vaccine distribution, especially amid concerns about Western countries potentially imposing travel restrictions if Africa’s response is perceived as inadequate.
He also noted the challenges related to vaccine access and the need for careful planning in vaccine deployment, including regulatory approval, supply chain logistics, and public communication to ensure vaccine acceptance.